Dialysis Machine Museum
Chronic hemodialysis only dates back to the 1940s. Before there was dialysis, chronic kidney disease was fatal. With the advent of the Kolff Rotating Drum dialysis machine in 1943, a new era of treatment for kidney failure began—which has saved hundreds of thousands of lives. Here, we share a brief history of dialysis from its start to the present.
Kolff Rotating Drum (1943)
The first practical artificial kidney was built during World War II by the Dutch doctor Willem Kolff. The Kolff kidney used a 20-meter long tube of cellophane sausage casing as a membrane. The tube of casing was wrapped around a slatted wooden drum. Run by an electric motor, the drum spun in a tank filled with dialysate.
To start a treatment, blood was drained from the patient into a sterile jug. Blood thinning drugs were added. The filled jug was hung on a post above the artificial kidney and linked to the casing that was wound around the wooden drum.
When the motor turned the drum, blood was pulled through by gravity to "prime" the casing (fill it with blood). Wastes from the blood diffused through the tubing into the dialysate in the tank. The cleansed blood went into a second sterile jug at the other end of the machine. An HD treatment took about 6 hours. When all of the blood had passed through the machine, the second jug was raised up to drain the cleaned blood back into the patient.
The Kolff kidney removed toxins from the blood. Since it ran at low pressure, it could not also remove excess fluid. (Modern machines remove both wastes and fluid.)
Kolff-Brigham Dialysis Machine: 1948
George Thorn, MD, of the Peter Bent Hospital in Boston, MA, invited Dr. Willem Kolff, MD, to work with Drs. Carl Walters and John Merrill to redesign the rotating drum machine. The new version was to be used to support the first U.S. kidney transplant program.
A sausage casing (cellulose) membrane was still wrapped around a drum. This time, it was connected to latex tubing that could be attached to the patient's bloodstream. As with the first model, the drum rotated in a vat of dialysate fluid.
The patient's blood was pushed through the device by the "Archimedes screw principle" and a pulsatile pump. A split coupling was built to connect the tubing to the membrane and keep the tubing and membrane from twisting. This coupling is at the inlet and outlet of the drum.
The membrane surface area could be adjusted by wrapping more or fewer loops of tubing around the drum. A Plexiglas™ hood was added to control the blood temperature.
This device was built by Edward Olson, an engineer, who produced forty more of the devices, which were shipped all over the world. The cost was $5,600 in 1950.
- Murphy WP Jr., Swan RC Jr., Walter CW, Weller JM, Merrill JP. Use of an artificial kidney. III. Current procedures in clinical hemodialysis. J Lab Clin Med. 1952 Sep; 40(3):436–44.
Skeggs Leonards Plate Dialyzer: 1948
Leonard Skeggs, PhD, and Jack Leonards, MD, built the first parallel flow artificial kidney at Case Western Reserve in Cleveland, OH. The kidney was designed to have a low resistance to blood flow and a surface area that could be adjusted.
The device had stacks of "sandwiches", each with two sheets of membrane between two rubber pads. This design reduced the blood volume that had to be in the dialyzer. It also ensured a uniform distribution of blood across the membrane for more efficiency. The device took a great deal of time to build, and often leaked. Wax was used to stop leaks.
The device had a very low resistance to blood flow and a single unit could be used with no blood pump. (If more than one of these units were used at a time, a blood pump was needed.) Dr. Skeggs was able to remove water from the blood by creating a siphon on the effluent of the dialysate. This seems to be the first use of negative pressure in dialysis.
Autoanalyzer - This technology was later adapted by Leonard Skeggs to do blood chemistries. It was called the SMA 12-60 Autoanalyzer.
Guarino and Guarino Artificial Kidney: 1952
This artificial kidney was made to reduce the amount of blood outside of the body and the need for a pump.
Guarino used cellulose acetate tubing. The dialysate went inside the tubing, while blood cascaded down the membrane from the top of the device. Metal tubing inside the membrane gave it support.
The artificial kidney did have a very low blood volume. However, it had limited use because there were concerns that dialysate might leak into the blood.
Pressure Cooker Artificial Kidney—Inouye & Engleberg: 1953
In 1948, Von Garrelts built a dialyzer by wrapping a cellulose acetate membrane around a core. The layers of membrane were held apart by rods. This was very bulky—and weighed over 100 pounds.
Dr. William Inouye took the concept and miniaturized it. He wrapped the membrane around a beaker and held the layers apart with fiberglass screening. He placed this "coil" into a Presto Pressure Cooker to enclose it and control the temperature. And, he made holes in the pot for the dialysate. By applying a vacuum to the dialysate leaving the pot, he was able to draw excess water out of the patient's blood. A blood pump was needed to overcome resistance in the device.
This device was used on patients. When it was used in a closed circuit, the exact amount of fluid removed could be determined.
- Inouye WY, Engelberg J. A simplified artificial dialyzer and ultrafilter. Surg Forum. Proceedings of the Forum Sessions, Thirty-ninth Clinical Congress of the American College of Surgeons, Chicago, Illinois, October, 1953; 4:438–42.
Travenol U200A Twin Coil (Kolff's "Orange Juice" kidney)—Kolff & Graham: 1956
This was the first commercially sold, disposable dialyzer. It was launched on October 30, 1956, by the Travenol Division of Baxter Laboratories, Inc., in Morton Grove, IL. The machine was a result of the efforts of Dr. Willem Kolff and William B. Graham, CEO of Baxter. The coil came with an inlet (arterial) bloodline, U200B, and an outlet (venous) bloodline, U200C. The coil and bloodlines sold for $59.00. The urea clearance was 140 mL/min. Since the priming volume was 1200 to 1400 mL, the priming blood volume for the patient was refrigerated between treatments.
- McBride P. Genesis of the Artificial Kidney. Baxter Healthcare; 45–46.
Kiil Dialyzer: 1960
These Kiil boards were built in Norway under the direction of Fred Kiil, MD. Three or more boards were used with two sheets of membrane sandwiched between each pair of boards. The membrane used was Cuprophan™. Grooves in the boards directed the blood between the layers of membrane—and the dialysate outside the membrane envelope—in opposite directions from one end of the boards to the other.
The priming volume was less than 300 cc. When a shunt was used, blood could be pumped through the device with blood pressure only. No blood pump was needed. Excess fluid was removed by the use of negative pressure on the dialysate effluent line.
This type of device was used for overnight, unattended HD, which was pioneered by Belding Scrbner, MD, and his group in Seattle, WA. A totally monitored dialysate system was needed to mix the dialysate and to control temperature and conductivity. This system was built by Albert Babb, PhD, while he was at the University of Washington.
- Kiil F. Development of parallel-flow artificial kidney. Acta Chir Scand. 1960; Suppl 253:142–150.
Drake-Willock Model 4002 with Kiil Dialyzer: Mid 1960s
In 1964, Charles Bernard Willock's young daughter invited a friend home. She brought along her father, Richard Drake, MD, a nephrologist—who told Willock about a patient who needed $30,000 for dialysis. The patient had sold his house to pay for the life-saving treatment, but his wife left him and took the money. Drake told Willock what was needed to do the treatments, and Willock designed a machine "in about an hour," per an article in The Oregonian. He built the prototype out of parts in his basement, spending only $250 for new parts.</>
A few months later, the first machine was used on a patient at Good Samaritan Hospital. Then Drake and Willock founded the Drake Willock Co. to build the machines in Milwaukie, OR. Five hundred machines were sold during their first year. In time, they were building about 300 machines a month, with international sales of more than $12 million in 1977, the year the company was sold.
Used with permission from iKidney.
Milton-Roy Model A—First Machine Used for Nocturnal Home Hemo: 1964
This Model A machine was built from a prototype "Mini-1" machine designed by Albert "Les" Babb for his best friend's daughter, Caroline Helm. It was called the Mini-1 because Dr. Babb had built a much larger one—"The Monster"—for the University of Washington before this smaller home version.
The Model A was built by the Milton Roy Company in St. Petersburg, Florida in 1964. It was designed to perform nocturnal home HD. The wooden veneer was used to give it a furniture look for home use. The Model A had automatic hot water (90° C) disinfection, automatic alarms, solid-state (diode) logic, and acoustic tile inside to reduce noise.
This machine became the first of a series of negative pressure single patient systems, culminating in the 9th generation, the Baxter Arena introduced in 2002.
- Twardowski, Zbylut J., Laudatio: Albert L. Babb, Hemodialysis International, Volume 7, Number 4, 2003.
Automatic PD Cycling Machine: 1964
S.T. Boen, C.M. Mion, F.T. Curtis and G. Shilipetar developed an automated device to do PD at home. It used a 40-liter glass bottle that was filled and sterilized at the University of Washington. The bottles were brought to a patient's home and returned to the hospital after use.
A cam cycler timer was used to meter the fluid into and out of the peritoneal cavity. A heater plate heated the fluid to body temperature, and the drain amount was measured.
Fred Boen, MD, used the "repeated puncture" method for access. A physician had to go to a patient's home and surgically place a 14F trocar in the abdomen. A helper was trained to remove the trocar after the treatment. Glass bottles were used for PD fluid until 1978, when bags came out.
- Boen ST, Mion CM, Curtis FK, Shilipetar G. Periodic peritoneal dialysis using the repeated puncture technique and an automatic cycling machine. Trans Am Soc Artif Intern Organs. 196; 10:408–14.
Mini II Hemodialysis Machine (mid 1960s)
On an airplane flight from Seattle to an East Coast conference in 1965, four innovators refined the design of the first home dialysis machine, which they had created a few months before. Dr. Scribner, Professor Babb, Lars Grimsrud, a grad student of Babb's, and Jack Cole, the first dialysis engineer, met around a coffee table in the back of the plane. They came up with the new design in about 4 hours. The Milton Roy Company in Florida made five of these new Mini-II machines for home dialysis trials. The mini-II proved successful and became the first commercial home dialysis machine.
Capillary Artificial Kidneys (Hollow Fiber Dialyzers): 1964–1967
Richard Stewart, MD, was given a grant to explore medical uses of cellulose acetate capillary fibers. His group thought the fibers could be used to make an artificial kidney. The original "Capillary Kidney" showed that wastes could be selectively removed from the blood, as well as excess water.
Stewart et al built larger versions for patient use. Their goal was that the device be as efficient as a "Twin Coil Dialyzer," but with a lower priming volume and better reliability.
The model shown here was first used at the University of Michigan in Ann Arbor, MI, and later at the Marquette School of Medicine in Milwaukee, WI. The "Capillary Artificial Kidney" (or hollow fiber dialyzer) has become the standard for HD today.
Travenol RSP: 1967
This fully integrated dialysis delivery system was developed by Travenol Laboratories for use by hospitals and for home dialysis. The initial cost of the system was $1,400, and over 3,500 of these devices were produced and used all over the world.
The machine required that the bath be mixed each time. The reservoir contained 120 liters of water and concentrate. Many centers used ordinary tap water. The treatment time was six hours and the patient was treated from one to three times a week depending on the condition of the patient.
This type of machine utilized the coil type dialyzer and it was later modified to use the hollow fiber dialyzer. The "Travenol RSP" is the term used to describe this hemodialysis system. It means single pass recirculating hemodialysis machine.
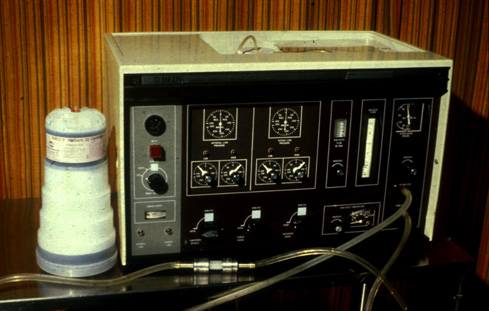
REDY Machine: 1973
The notion of regenerating dialysate instead of discarding it after use was first suggested in 1966, reported Malcolm Roberts. “Sorbents consisting of zirconium phosphate, hydrated zirconium oxide, and carbon combined with urease were placed in a cartridge and, after completion of animal and human studies, the system was placed on the commercial market in 1973.”1 This REDY (REgeneration of DialYsate) machine used sorbents to remove solutes and toxins from used dialysate, purify and reconstitute it, and send it back through the dialyser. Water use per treatment dropped from about 500/L to about 6L.
The REDY was, for its time, quite successful, and sustained home HD for more than a decade. In Australia and New Zealand, the REDY was credited with “saving” home HD.2 Cost was its main Achilles heel, and single pass systems using HFAK dialysers soon priced the REDY out of the market for maintenance HD, though it kept a presence into the mid 90’s for inpatient treatment of acute kidney injury. Currently, the sorbent concept is making a comeback, especially by those attempting to develop a wearable kidney.
A review of sorbents can be found in Agar JWM. Understanding Sorbent Dialysis Systems. Nephrology (Carlton). June 2010. 15(4): 406-411.
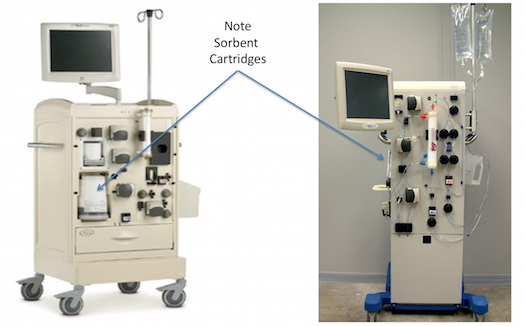
The RenalSolutions Inc Allient and Eagle Sorbent Systems: 2007-2012
In the first few years of the 21st century, RenalSolutions Inc (RSI). made a bold attempt to bring back the REDY concept in a new and improved system, called the “Allient” that was released briefly in 2007, as the “Eagle”. The sorbent cartridge was improved to remove some of the concerns of the REDY, such as potential aluminium release and contamination of the dialysate. A novel, vacuum-driven, drop-in disposable cassette created an externalised wet surface system that minimised the risks to internal circuitry.
After a few months, RSI was purchased by Fresenius with an intent to use the technology in a smaller, portable system: the Portable Artificial Kidney (or PAK). The PAK stalled in 2011/12 when the sorbent column released small amounts of sodium into the dialysate, which raised some concerns at the FDA. The PAK returned to the drawing board to resolve the issue…and has not (yet) reappeared in any prototype or commercial system.
-
Nephrology. August 1998. 4(4) 275-278 ↩
-
Agar JWM, George C, Kerr PG. Dialysis: History, Development and Promise. (Eds: Ing TS, Rahman MA, Kjellstrand C M.) - Book Chapter: History of Dialysis – Australia and New Zealand. Publisher ‘World Scientific Press’: ISBN-10: 9814289752 | ISBN-13: 978-9814289757 ↩
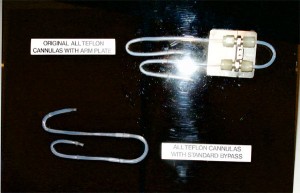
Teflon Dialysis Cannulas (Shunts)
Belding Scribner, MD, at the University of Washington in Seattle, WA, asked Wayne Quinton, a biomedical engineer, to help him develop a permanent access to the blood stream for patients who had kidney failure. Scribner was convinced that he could chronically treat a patient with kidney failure if a permanent access was available.
Quinton developed a vessel access using Teflon™ tubing. This material was successful because of its non-stick properties that prevented the blood from adhering to it and clotting.
They were able to demonstrate that a permanent access was possible and that they could chronically dialyze a kidney failure patient.
Later they would use silicone tubing for the external segment in order to act as a shock absorber and to provide more comfort and safety for the patient. This device was worn by a chronically-dialyzed kidney patient.
- Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960 Apr 10-11; 6: 104-13.
Drake-Willock PD Cycler Machine: 1970s
This PD machine (3 bottles, 2 pumps) was made by Drake Willock. It’s identified as Model 6010. It incorporated an RO inside to make the peritoneal solution, so the 40 liter bottles were no longer needed.
The demise of this unit was the introduction of plastic bags of PD solution and CAPD by Baxter in the late 1970s.
Flat Plate (or Parallel Plate) Dialyzer
This type of dialyzer used sheets of membrane mounted on top of plastic support screens, and stacked in layers. Dialysate would flow between some of the pairs of membranes, and blood between other pairs. The design was lo-cost, and allowed for very little resistance to flow, so fewer anti-clotting medications were needed. In time, this model was entirely replaced by the hollow fiber dialyzer.
Travenol Laboratories Ultra Flo - II: 1974
This coil (product code 5M1755) was first produced by Travenol Laboratories, Inc., in 1974. It used a single coil of tubular Cuprophan membrane 210 mm wide and had a surface area of 1.4 m2. At a blood flo-rate of 200 mL/min, the dialyzer had clearances of urea of 133 mL/min, creatinine of 115 mL/min, and Vitamin B12 of 30 mL/min. At a venous pressure of 100 mmHg, the ultrafiltration rate was 487 mL/hr.
Travenol Laboratories Inc. Ultra Flo - II brochure number 8-19-3-147AA, September 1974.
CF 1200 and CF 1500 capillary flow dialyzers: 1977
These dialyzers first appeared in Dialysis and Transplant in August 1978. The capillaries were made of thin-wall (16 micron) Cuprophan fibers. At a 200 mL/min blood flo-rate, the dialyzer clearances were:
- CF1200: Urea - 160 mL/min, Creatinine - 140 mL/min, Vitamin B12 - 30 mL/min; Kuf = 2.5 mL/hr/mmHg (TMP)
- CF1500: Urea - 170 mL/min, Creatinine - 130 mL/min, Vitamin B12 - 25 mL/min; Kuf = 3.5 mL/hr/mmHg (TMP)
Travenol Laboratories, Inc., sales brochure for CF capillary flow dialyzers, May 1977.
CD 1000 and CD 1400 coil dialyzers: 1978
These dialyzers represented the culmination of 20 years of coil development for Travenol Laboratories, Inc. They used a 210 mm wide Cuprophan membrane. Both had clear cases to make visualization easier during rinsing. Having both dialysate ports on the bottom reduced splashing in the dialyzing compartment. At a blood flo-rate of 200 mL/min, the urea clearance for the CD 1000 was 110 mL/min and for the CD 1400 was 130 mL/min. At a mean coil pressure of 200 mmHg, the CD 1000 had a UFR of 450 mL/hr and the CD 1400 had a UFR of 700 mL/hr.
- Travenol CD coil dialyzer – the product of 20 years experience (advertisement), Dialysis and Transplantation, February 1978.
C-DAK Artificial Kidneys (Hollow Fiber Dialyzers)
The first presterilized, ready to use "Capillary Artificial Kidney" was made with a new capillary membrane called Cuprophan™.
The dialysis community was looking for artificial kidneys with increased efficiency. The way this objective was accomplished was by using more fibers in the device. As a result, the size of the artificial kidney began to grow. Later models of this type of device would utilize a thinner wall membrane that would allow for fewer fibers and a smaller size artificial kidney.
Automated Peritoneal Dialysis Cycler
The Baxter (Travenol Laboratories back then) "Automated Peritoneal Dialysis Cycler" introduced in 1984. When patients got tired of doing their own exchanges, Baxter automated the process so it would all happen at night. No-one called it the Automated Peritoneal Dialysis Cycler—too many words. It was called simply the PacX because the tubing setup made a X on the front panel.
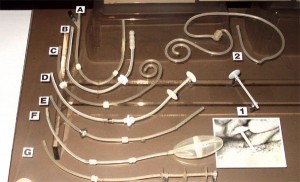
Peritoneal Dialysis Catheters
- The Goldberg catheter was designed to prevent migration by the inflation of a small balloon in the distal portion of the catheter. Inflation was achieved by pumping air into the side arm on the proximal end of the catheter.
- This unique peritoneal catheter contains a housing around the distal end of the catheter to prevent omentum interference. There are two rings inside the housing to prevent collapse. It is called the "Valli" catheter and was manufactured in Italy.
- This curled catheter has a weight at the distal end of the catheter to help keep the catheter down into the pelvic gutter. It is an early version of the Tecnkhoff curled catheter that uses Dacron™ cuffs.
- This is a prototype of a catheter with a silver collar designed to be placed at the exit site. Silver was selected because of its antibacterial properties.
- This is a prototype catheter with a stainless steel collar designed to secure the exit site to prevent infection.
- The Valli catheter was designed to prevent omental interference.
- Early prototype of the Toronto Western catheter.
Di Paolo N, Patrini G, Garosi G, Buoncristiani U, Brardi S, Monaci G. A new self-locating peritoneal catheter. Perit Dial Int. 1996; 16: 623-627.